What Makes Nitrogen In The Atmosphere Unavailable For Use By Living Organisms
N2 → NH4 +
Nitrogen (N) is an essential component of Dna, RNA, and proteins, the building blocks of life. All organisms require nitrogen to alive and grow. Although the bulk of the air nosotros breathe is N2, most of the nitrogen in the atmosphere is unavailable for use past organisms. This is because the strong triple bond between the N atoms in North2 molecules makes it relatively inert, or unreactive, whereas organisms need reactive nitrogen to exist able to incorporate it into cells. In club for plants and animals to be able to use nitrogen, Nii gas must first exist converted to more than a chemically available form such equally ammonium (NH4 +), nitrate (NO3 -), or organic nitrogen (e.g., urea, which has the formula (NH2)2CO). The inert nature of Nii means that biologically available nitrogen is often in brusk supply in natural ecosystems, limiting plant growth.
Nitrogen is an incredibly versatile element, existing in both inorganic and organic forms as well as many different oxidation states. The motion of nitrogen between the atmosphere, biosphere, and geosphere in different forms is called the nitrogen cycle (Figure 1), i of the major biogeochemical cycles. Similar to the carbon cycle, the nitrogen cycle consists of various reservoirs of nitrogen and processes by which those reservoirs exchange nitrogen (annotation the arrows in the effigy). (Run into The Carbon Cycle module for more information.)
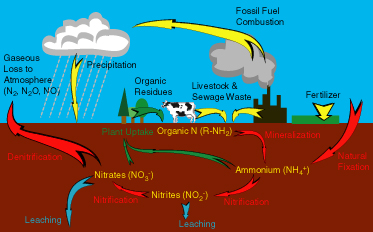
Processes in the nitrogen bicycle
V main processes cycle nitrogen through the biosphere, atmosphere, and geosphere: nitrogen fixation, nitrogen uptake through organismal growth, nitrogen mineralization through decay, nitrification, and denitrification. Microorganisms, particularly bacteria, play major roles in all of the main nitrogen transformations. Considering these processes are microbially mediated, or controlled by microorganisms, these nitrogen transformations tend to occur faster than geological processes like plate motion, a very ho-hum, purely physical process that is a role of the carbon wheel. Instead, rates are afflicted by environmental factors that influence microbial activity, such as temperature, moisture, and resource availability.
Comprehension Checkpoint
Processes within the nitrogen cycle progress at a _______ charge per unit than geological processes like plate motion.
Nitrogen fixation
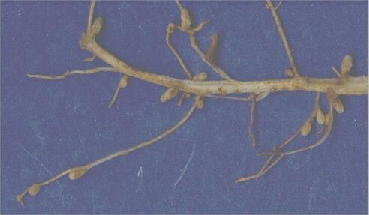
Nitrogen fixation is the process wherein N2 is converted to ammonium, or NH4 +. This is the only manner that organisms tin can attain nitrogen directly from the atmosphere; the few that can practice this are called nitrogen-fixing organisms. Certain bacteria, including those among the genus Rhizobium, are able to fix nitrogen (or catechumen information technology to ammonium) through metabolic processes, analogous to the fashion mammals catechumen oxygen to CO2 when they breathe. Nitrogen-fixing leaner often course symbiotic relationships with host plants. This symbiosis is well-known to occur in the legume family of plants (e.1000., beans, peas, and clover). In this relationship, nitrogen-fixing bacteria inhabit legume root nodules (Figure 2) and receive carbohydrates and a favorable surround from their host institute in exchange for some of the nitrogen they set up. There are besides nitrogen-fixing leaner that exist without found hosts, known as free-living nitrogen fixers. In aquatic environments, bluish-green algae (really a bacteria called cyanobacteria) are an of import free-living nitrogen fixer.
In addition to nitrogen-fixing leaner, high-energy natural events such as lightning, wood fires, and fifty-fifty hot lava flows can crusade the fixation of smaller, but pregnant, amounts of nitrogen. The high energy of these natural phenomena can break the triple bonds of N2 molecules, thereby making individual N atoms available for chemical transformation.
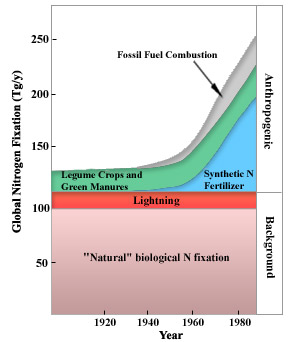
Inside the last century, humans have go every bit important a source of stock-still nitrogen as all natural sources combined. Burning fossil fuels, using synthetic nitrogen fertilizers, and cultivating legumes all fix nitrogen. Through these activities, humans have more than doubled the corporeality of fixed nitrogen that is pumped into the biosphere every yr (Figure 3), the consequences of which are discussed below.
Nitrogen uptake
NH4 + → Organic N
The ammonium (NHfour +) produced by nitrogen-fixing bacteria is normally quickly taken up by a host plant, the leaner itself, or some other soil organism and incorporated into proteins and other organic nitrogen compounds, like Deoxyribonucleic acid. When organisms nearer the top of the food chain (similar us!) eat, we are taking upward nitrogen that has been fixed initially by nitrogen-fixing bacteria.
Nitrogen mineralization
Organic N → NH4 +
After nitrogen is incorporated into organic matter, it is ofttimes converted back into inorganic nitrogen by a process chosen nitrogen mineralization, otherwise known equally disuse. When organisms die, decomposers (such as bacteria and fungi) consume the organic matter and pb to the process of decomposition. During this process, a significant amount of the nitrogen contained within the dead organism is converted to ammonium. Once in the form of ammonium, nitrogen is bachelor for use by plants or for further transformation into nitrate (NO3 -) through the process called nitrification.
Nitrification
NHfour + → NO3 -
Some of the ammonium produced by decomposition is converted to nitrate (NOthree -) via a process chosen nitrification. The leaner that carry out this reaction gain energy from it. Nitrification requires the presence of oxygen, so nitrification can happen just in oxygen-rich environments like circulating or flowing waters and the surface layers of soils and sediments. The procedure of nitrification has some of import consequences. Ammonium ions (NH4 +) are positively charged and therefore stick (are sorbed) to negatively charged clay particles and soil organic matter. The positive accuse prevents ammonium nitrogen from beingness washed out of the soil (or leached) by rainfall. In contrast, the negatively charged nitrate ion is non held by soil particles and so can exist washed out of the soil, leading to decreased soil fertility and nitrate enrichment of downstream surface and groundwater.
Denitrification
NO3 - → Ntwo+ North2O
Through denitrification, oxidized forms of nitrogen such as nitrate (NO3 -) and nitrite (NO2 -) are converted to dinitrogen (N2) and, to a lesser extent, nitrous oxide gas (NOtwo). Denitrification is an anaerobic procedure that is carried out by denitrifying leaner, which convert nitrate to dinitrogen in the following sequence:
NO3 - → NOtwo - → NO → N2O → N2.
Nitric oxide and nitrous oxide are gases that have environmental impacts. Nitric oxide (NO) contributes to smog, and nitrous oxide (Due north2O) is an important greenhouse gas, thereby contributing to global climate change.
In one case converted to dinitrogen, nitrogen is unlikely to exist reconverted to a biologically available form because information technology is a gas and is rapidly lost to the atmosphere. Denitrification is the only nitrogen transformation that removes nitrogen from ecosystems (essentially irreversibly), and it roughly balances the amount of nitrogen fixed by the nitrogen fixers described in a higher place.
Comprehension Checkpoint
Which process returns nitrogen gas to the atmosphere?
Homo alteration of the N bicycle and its environmental consequences
Early in the 20th century, a German scientist named Fritz Haber figured out how to brusque-circuit the nitrogen cycle by fixing nitrogen chemically at high temperatures and pressures, creating fertilizers that could be added direct to soil. This applied science spread rapidly over the 20thursday century, and, along with the advent of new crop varieties, the use of synthetic nitrogen fertilizers led to an enormous smash in agricultural productivity. This agricultural productivity has helped us to feed a rapidly growing world population, but the increase in nitrogen fixation has had some negative consequences as well. While the consequences are maybe not as obvious as an increase in global temperatures (meet our Data Analysis and Interpretation module) or a hole in the ozone layer (run into The Exercise of Science module), they are merely equally serious and potentially harmful for humans and other organisms.
Why? Non all of the nitrogen fertilizer applied to agronomical fields stays to nourish crops. Some is washed off of agricultural fields by rain or irrigation water, where it leaches into surface water or groundwater and can accumulate. In groundwater that is used as a drinking h2o source, backlog nitrogen can atomic number 82 to cancer in humans and respiratory distress in infants. The US Ecology Protection Agency has established a standard for nitrogen in drinking water of 10 mg per liter nitrate-North. Unfortunately, many systems (specially in agronomical areas) already exceed this level. By comparison, nitrate levels in waters that have not been altered past man activity are rarely greater than i mg/L. In surface waters, added nitrogen can pb to food over-enrichment, particularly in littoral waters receiving the inflow from polluted rivers. This food over-enrichment, too called eutrophication, has been blamed for increased frequencies of littoral fish-kill events, increased frequencies of harmful algal blooms, and species shifts inside coastal ecosystems.
Reactive nitrogen (like NOiii - and NH4 +) nowadays in surface waters and soils, can also enter the temper every bit the smog-component nitric oxide (NO) which is a component of smog, and besides equally the greenhouse gas nitrous oxide (Due north2O). Eventually, this atmospheric nitrogen tin be diddled into nitrogen-sensitive terrestrial environments, causing long-term changes. For example, nitrogen oxides comprise a pregnant portion of the acidity in acid rain, which has been blamed for forest death and pass up in parts of Europe and the northeastern U.s.. Increases in atmospheric nitrogen deposition take too been blamed for more subtle shifts in dominant species and ecosystem function in some forest and grassland ecosystems. For example, on nitrogen-poor serpentine soils of northern Californian grasslands, constitute communities accept historically been express to native species that tin can survive without a lot of nitrogen. There is now some bear witness that elevated levels of atmospheric Due north input from nearby industrial and agronomical evolution take allowed invasion of these ecosystems by non-native plants. As noted before, NO is too a major factor in the formation of smog, which is known to cause respiratory illnesses like asthma in both children and adults.
Currently, much inquiry is devoted to understanding the effects of nitrogen enrichment in the air, groundwater, and surface water. Scientists are also exploring alternative agricultural practices that volition sustain high productivity while decreasing the negative impacts caused past fertilizer utilize. These studies not only aid us quantify how humans have altered the natural world, but increment our understanding of the processes involved in the nitrogen cycle as a whole.
Summary
Although the majority of the air we breathe is N2, molecular nitrogen cannot be used straight to sustain life. This module provides an overview of the nitrogen bike, one of the major biogeochemical cycles. The five principal processes in the bicycle are described. The module explores human impact on the nitrogen cycle, resulting in not only increased agricultural production simply also smog, acid pelting, climate change, and ecosystem upsets.
Key Concepts
-
The nitrogen bike is the ready of biogeochemical processes by which nitrogen undergoes chemical reactions, changes form, and moves through difference reservoirs on Earth, including living organisms.
-
Nitrogen is required for all organisms to live and grow because it is the essential component of DNA, RNA, and protein. Nevertheless, most organisms cannot use atmospheric nitrogen, the largest reservoir.
-
The 5 processes in the nitrogen wheel – fixation, uptake, mineralization, nitrification, and denitrification – are all driven by microorganisms.
-
Humans influence the global nitrogen cycle primarily through the use of nitrogen-based fertilizers.
What Makes Nitrogen In The Atmosphere Unavailable For Use By Living Organisms,
Source: https://www.visionlearning.com/en/library/Earth-Science/6/The-Nitrogen-Cycle/98
Posted by: baltzcoonly63.blogspot.com


0 Response to "What Makes Nitrogen In The Atmosphere Unavailable For Use By Living Organisms"
Post a Comment